Breadcrumb
Three Oxford chemistry groups collaborate on amorphous calcium carbonate
'Geometrically frustrated interactions drive structural complexity in amorphous calcium carbonate' – a paper co-authored by a team of scientists including Christ Church’s Professor Dirk Aarts – has been published in Nature Chemistry.

Amorphous calcium carbonate (ACC) is one of nature’s important building materials. It lacks an ordered atomic structure, making it amorphous, or non-crystalline. This complex structure makes it valuable to marine organisms that use it to form structures such as shells. However, working out the best way to understand the structure of ACC, and rationalising why it forms so readily, have both remained longstanding open questions.
This new study combines experimental data with advanced computer simulation techniques to answer these questions. The research draws on the work of DPhil student Tom Nicholas and former DPhil student Adam Stones, and is the result of a collaboration between the Aarts, Deringer and Goodwin groups in the Oxford University Department of Chemistry and two US teams.
I’m honoured to have been involved in such an exciting topic.
I’m honoured to have been involved in such an exciting topic.
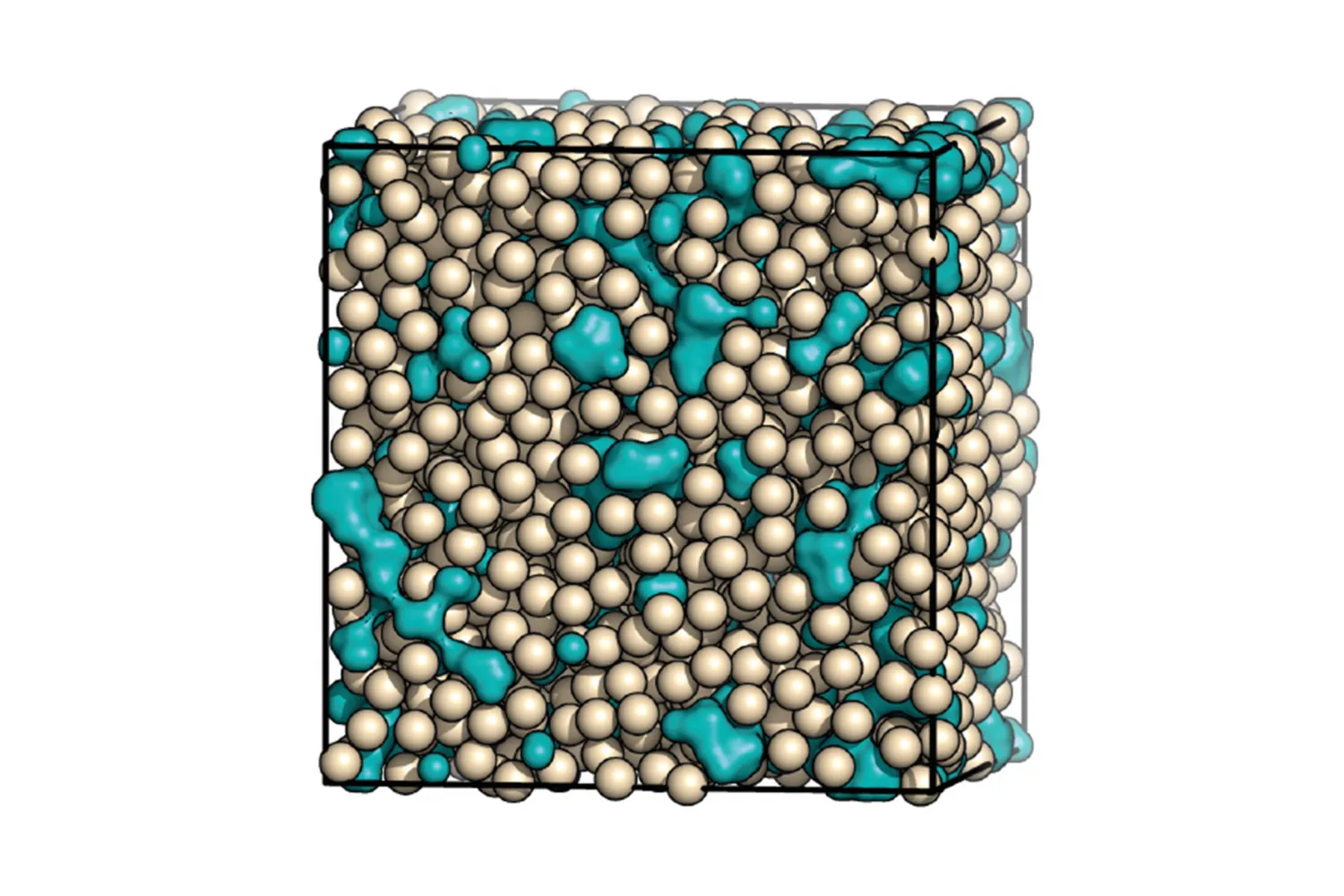
The team detailed their investigation: ‘To better understand the structure and resulting metastability of ACC, the first aim was to generate a high-quality atomistic model of ACC, which was done by using state-of-the-art interatomic potentials to guide interpretation of diffuse X-ray scattering data. The main features of the model are a disordered network of calcium cations, linked by carbonate anions of opposite charge, and interspersed water channels, visible in the image above (a “blue cheese” model).
‘With this new structural model in hand, we extracted the effective interactions between the calcium ions using a recently developed “inversion” method. We found that the effective interaction between the calcium ions has two attractive minima, arising from the two different ways in which carbonate anions can bridge calcium cations. These competing length scales lead to geometrically frustrated ordering – there being no simple way for the ions to pack into an extended structure – explaining the amorphous nature and the relative stability of ACC.’
Responding to the news that the team’s research would be published in Nature Chemistry, Professor Aarts said: ‘It’s great to see our inversion method, which we developed a couple of years ago to better understand interactions between colloidal particles, used in a completely different field of chemistry. I’m honoured to have been involved in such an exciting topic.’
The paper was published this afternoon and is available via Open Access at: https://www.nature.com/articles/s41557-023-01339-2.
Professor Aarts is Senior Censor and a tutor in Physical Chemistry at Christ Church and leads the Oxford Colloid Group. Further information about his research can be found on his online profile.
Other Christ Church news